Key points
- Herbicide persistence and carryover between seasons can damage rotational crops.
- Soil testing can measure herbicide residues, but the results can be difficult to interpret. Critical damage thresholds are rarely available and depend on soil type.
- The project developed over 80 new herbicide-soil-crop critical damage thresholds. These can help growers and advisers interpret soil and plant tissue tests.
- Rainfall (soil moisture) and soil type are key drivers of herbicide dissipation and breakdown in soil. Pre-sowing soil tests for herbicide residues after low rainfall can guide crop decisions and reduce damage risk.
The challenge
Herbicides with soil residual activity are widely used to manage herbicide-resistant weeds but can persist in the soil for 12 to 24 months, potentially affecting subsequent crops. However, data on residue levels and crop impacts in Australian cropping soils are limited.
A major challenge is the lack of established toxicity thresholds, which define the herbicide concentration in soil that harms crops. While commercial labs offer testing, results are hard to interpret because tests measure total residues, not bioavailability – the portion accessible to plants – which varies with soil type and chemistry.
Environmental factors such as frost, soil micronutrient levels and crop tolerance further complicate predictions. Residue persistence is also influenced by soil texture, organic matter, temperature and moisture, with dry conditions slowing breakdown and increasing risk. Some herbicides remain in soil at biologically active concentrations, affecting sensitive crops long after application.
Without clear thresholds, it is difficult to assess yield risks. Herbicide labels provide only general guidance and lack site-specific recommendations. Residue symptoms can be misdiagnosed as other crop stressors, such as nutrient deficiencies, drought or pests, further complicating management decisions.
Growers and consultants need reliable tools to assess herbicide residues and their risks. Addressing this gap requires improved testing methods, better interpretation of residue data and clear toxicity thresholds to help farmers make informed decisions and minimise crop damage.
Our research
Phytotoxicity thresholds
Our research aimed to define phytotoxicity thresholds for crops exposed to soilborne herbicide residues through glasshouse and field experiments. Dose-response experiments were conducted using sand (to reflect maximum herbicide bioavailability) and a range of soils were spiked with increasing concentrations of herbicides.
The herbicides investigated included diuron, clopyralid, imazapyr, pyroxasulfone, trifluralin and propyzamide. Wheat, barley, canola, lentil, lupin, field pea, chickpea and faba bean were grown in treated soils under controlled conditions for 28 days.
After 28 days, shoots and roots were separated, cleaned and assessed. Scanners measured root length and branching, while shoot biomass was dried and weighed.
Leaf tissue testing confirmed herbicide-related growth reductions. Toxicity thresholds were determined at ED20, the concentration causing a 20% reduction in biomass. Selected shoot tissue samples at ED20 levels were also analysed for herbicide residues. Additional dose-response data were sourced from published literature and databases across soil and crop types.

Herbicide carryover
Field surveys and designed field experiments were conducted in the central Western Australian wheatbelt, at Minnipa on the Eyre Peninsula (South Australia) and at Birchip in the Victorian Mallee. Herbicide carryover of imazapyr, clopyralid, diuron, and pyroxasulfone was measured across different soils and climates over two cropping seasons. More than 300 composite soil samples were collected from multiple paddock locations at 0-10 cm and 10-30 cm depths to assess vertical and spatial variation in residue concentrations.
In cases of poor crop growth, leaf tissue was also analysed. Herbicide concentrations in soil and plant tissue were compared with known toxicity thresholds to determine whether residue levels were high enough to affect crop health.
Research findings
Critical thresholds
Glasshouse dose-response experiments in sand identified the minimum levels of diuron, imazapyr, clopyralid, pyroxasulfone, trifluralin and propyzamide required to reduce crop biomass for wheat, barley, canola, lentil, lupin, field pea, chickpea or faba bean. Example results are given in Table 1. The thresholds are available in the final project report.
Follow-up dose-response experiments in soil demonstrated that residue concentration thresholds were generally higher in soil than in sand due to the influence of soil properties on sorption and bioavailability. The results reinforced that soil characteristics play a crucial role in determining herbicide persistence and crop impact.
Table 1. Imazapyr toxicity thresholds (ng/g) for 20% shoot dry weight reductions (ED20).
| Crop | Sand (0% clay) | Minnipa, SA Soil (6% clay, pH 7.8, OC = 1.0%) | Birchip, Vic Soil (14% clay, pH 7.4, OC = 1.1%) |
|---|---|---|---|
| Lentil | >30 | >30 | >30 |
| Field pea | 21 | >30 | >30 |
| Lupin | 19 | >30 | >30 |
| Chickpea | >30 | >30 | >30 |
| Faba bean | 3.7 | N/A | N/A |
| Wheat | 1.0 | 1.3 | 4.2 |
| Barley | 0.2 | 1.7 | 5.5 |
| Canola | 0.1 | 3.9 | 6.2 |
Herbicide residue variability and sampling advice
In the field experiments, residue persistence of imazapyr, clopyralid, diuron and pyroxasulfone depended heavily on rainfall. Less than 100 mm in the 180 days after application led to greater herbicide retention in soil and a higher risk to subsequent crops.
Herbicide residues were highest in the top 10 cm of soil, and concentrations varied significantly within paddocks, differing by a factor of five to ten over a 50 m distance.
Initial soil testing should focus on 0-10 cm, however, to fully understand herbicide movement, samples should be collected at 0-10 cm, 10-20 cm, and 20-30 cm. In cases where herbicides may leach deeper, such as when soil inversion is planned or where the soil profile has a sharp texture contrast, deeper sampling is recommended.
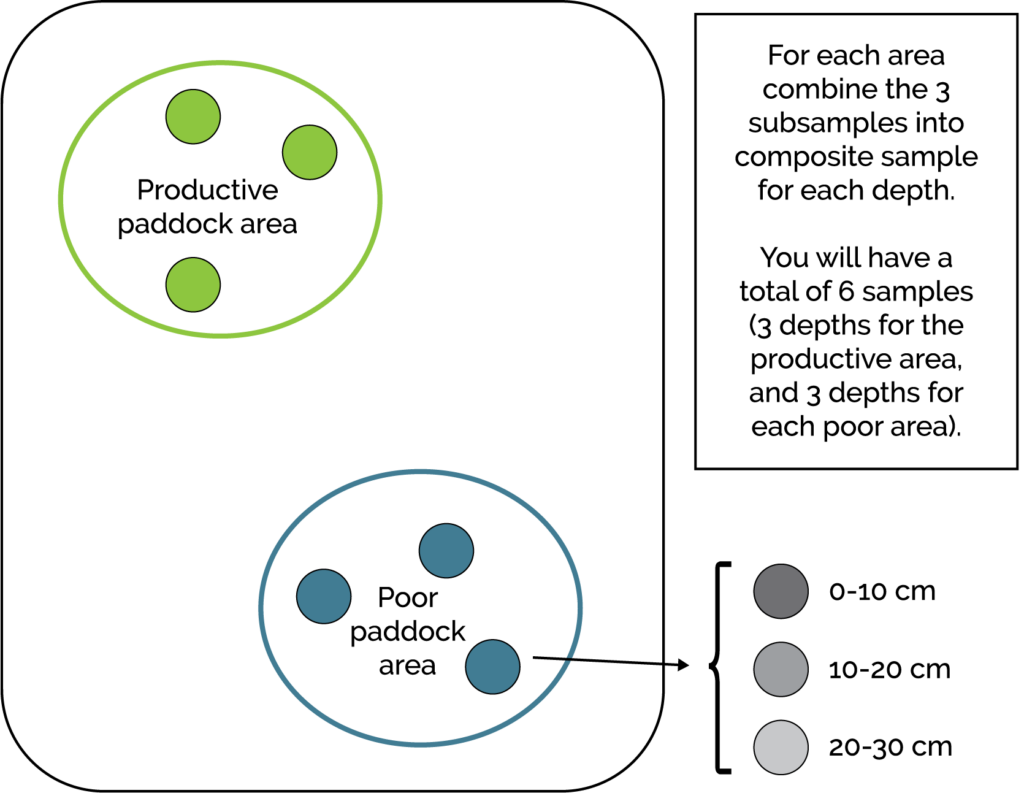
To address variability across the paddock, growers should sample at least two locations per paddock, ideally from both high- and low-productivity areas. In dune-swale systems, sampling should include dunes, swales, and seepage zones. Each laboratory submission should contain at least three composite subsamples from the targeted area (Figure 2).
Growers and advisors should discuss testing requirements with commercial service providers, as some toxicity thresholds may be below standard laboratory reporting limits. In these cases, a test may indicate that herbicide residue was not detected, despite residues being present at biologically active levels.
This is particularly relevant for chemical classes such as sulfonylureas, imidazolinones and picolinic acids like clopyralid. Where herbicide history is uncertain, such as when acquiring or leasing new land, multiresidue analysis is recommended. Additional phytotoxicity thresholds for herbicide-crop combinations are provided in the final project report.
Soil and plant tissue analysis for herbicide residues is not a replacement for using herbicides according to label requirements, but can provide additional context for decision making.
Significance of findings
Agronomists and growers will be able to use soil and leaf tissue testing to help diagnose herbicide damage for the herbicides and crops investigated in this project. The research confirms that leaf tissue testing can help to identify the cause of poor crop growth, reducing misdiagnoses and avoiding unnecessary expenses.
Soil and leaf tissue herbicide residue test results improve understanding of how herbicides persist and move through the environment. When combined with field observations, test results can help diagnose poor crop growth and support more confident decisions about herbicide use and crop selection. This is especially important when the same mode of action is used over consecutive seasons, to ensure residues are not building up to levels that restrict crop rotation options. Testing also allows growers to select crop species or cultivars that tolerate existing residues, reducing the risk of yield loss or crop failure.
The framework developed in this project can now be used by researchers and industry to explore the fate and potential effects of other herbicides, including new products, to help prevent unintended consequences of their use.

Next steps
Pilot-scale testing of the soil and plant tissue testing framework is now needed to validate our approach for predicting herbicide residue damage across a wider cropping area. Further research is also needed to understand how early biomass reductions relate to yield impacts. As new herbicides with different chemistry and behaviour are registered, data on toxicity thresholds and bioavailability will be essential to support safe and effective use.